A summary of commonly used water treatment methods for different water qualities!
Within the environmental water treatment sector, commonly employed water treatment methods generally include: (1) sediment filtration; (2) water softening; (3) activated carbon adsorption; (4) deionisation; (5) reverse osmosis; (6) ultrafiltration; (7) distillation; (8) ultraviolet disinfection; and (9) biochemical processes. The principles and functions of these water treatment methods are outlined below.

I. Sediment Filtration Method
The purpose of sediment filtration is to remove suspended particulate matter or colloidal substances from the water source. If these particles remain unremoved, they may damage other precision filtration membranes used in dialysis water systems or even cause blockages in water pathways. This constitutes the oldest and simplest method of water purification. Consequently, it is frequently employed as a preliminary treatment in water purification processes. Where necessary, additional filters may be incorporated within pipework to eliminate larger impurities.
A diverse array of filter media is utilised for retaining suspended particulate matter, including mesh filters, granular media (such as quartz sand), and membrane filters. Any particles larger than the pore size of these media will be effectively retained.
One issue with sediment filtration warrants attention: as particles accumulate through continuous interception, bacteria may proliferate on their surfaces and release toxic substances through the filter, causing pyrogenic reactions. Therefore, filters must be replaced regularly. As a rule, replacement is required when the pressure differential between inlet and outlet water increases to five times the original value.

II. Hard Water Softening Method (Water Softening Equipment)
Hard water softening employs ion exchange technology. This process utilises cation exchange resin to replace calcium and magnesium ions in hard water with sodium ions, thereby reducing the concentration of calcium and magnesium ions in the water source. The softening reaction is as follows: Ca²⁺ + 2Na⁻-EX → Ca-EX²⁺ + 2Na⁺ Mg²⁺ + 2Na⁻-EX → Mg-EX²⁺ + 2Na⁺ Ion exchange resins commercially available are spherical synthetic organic polymeric electrolytes.
Should water treatment omit cationic softening, calcium and magnesium deposits will accumulate on reverse osmosis membranes, diminishing their efficacy or even causing damage. Concurrently, patients become susceptible to hard water syndrome. Water softeners may also foster bacterial proliferation, necessitating backwashing functionality within the equipment. Periodic backwashing is required to prevent excessive impurity adsorption.
III. Activated Carbon Filters
Activated carbon is produced by carbonising materials such as wood, wood chips, fruit pits, coconut shells, coal, or petroleum residues through high-temperature pyrolysis. Following production, it undergoes activation using hot air or steam. Its primary function is to remove chlorine, chloramines, and other soluble organic compounds with molecular weights ranging from 60 to 300 daltons. Activated carbon exhibits a granular surface with a highly porous interior, containing numerous capillary pores measuring approximately 10 nanometres to 1 micrometre in diameter. One gram of activated carbon possesses an internal surface area of 700 to 1400 square metres, with adsorption occurring on both the inner surfaces of these capillaries and the granular surface itself.

IV. Deionisation Method
The deionisation method aims to remove dissolved inorganic ions from water, employing the same ion exchange resin principle as water softeners. Two types of resin are utilised: cation exchange resin and anion exchange resin. Cation exchange resin utilises hydrogen ions (H+) to exchange cations, while anion exchange resin employs hydroxide ions (OH-) to exchange anions.
Once the adsorption capacity of these resins is exhausted, they require regeneration. Cation exchange resin requires strong acid for regeneration; conversely, anion exchange resin requires strong alkali. Should anion exchange resin be depleted without regeneration, fluoride—the weakest adsorbed ion—gradually appears in dialysis water, causing osteomalacia, osteoporosis, and other bone disorders. Conversely, if cation exchange resin is exhausted, hydrogen ions enter the dialysis water, increasing its acidity. Thus, the efficacy of deionisation must be continuously monitored. This is generally determined by the resistivity or conductivity of the water. It is worth noting that the ion exchange resins used in deionisation can also cause bacterial proliferation, leading to bacteraemia.
V. Reverse Osmosis
Reverse osmosis effectively removes dissolved inorganic substances, organic matter, bacteria, pyrogens, and other particulates from water, constituting the most critical stage in dialysis water treatment.
Reverse osmosis achieves purification at the ionic level. Common semi-permeable membrane materials for reverse osmosis water treatment include cellulose-based membranes, aromatic polyamides, polyimide, or polyfuranes. Structural configurations encompass spiral-wound, hollow-fibre, and tubular types.
Failure to implement adequate pretreatment prior to reverse osmosis may lead to fouling of the permeate membrane by accumulated contaminants such as calcium, magnesium, and iron ions, thereby diminishing reverse osmosis performance. Certain membranes (e.g., polyamide) are susceptible to degradation by chlorine and chloramine compounds. Consequently, pretreatment stages including activated carbon filtration and water softening must precede reverse osmosis membrane operation. This process step is therefore highly recommended when preparing dialysate water for haemodialysis.

VI. Ultrafiltration
Ultrafiltration shares similarities with reverse osmosis in employing semi-permeable membranes, yet it cannot control ion removal due to its larger pore size (approximately 10-200 Å). It effectively excludes bacteria, viruses, pyrogens, and particulate matter, but cannot filter water-soluble ions.
Ultrafiltration primarily serves as a pre-treatment for reverse osmosis to prevent bacterial contamination of the reverse osmosis membrane. It may also be employed as the final stage in water treatment to prevent bacterial contamination of upstream water within the pipework. The effectiveness of ultrafiltration membranes is typically assessed by the pressure differential between the inlet and outlet water. Similar to activated carbon, backwashing is routinely used to remove impurities adhering to the membrane surface.
VII. Distillation
Distillation is an ancient yet effective water treatment method capable of removing all non-volatile impurities. However, it cannot eliminate volatile contaminants. It requires substantial storage tanks, which, along with the associated pipework, represent significant sources of contamination. Consequently, this method is not currently employed for treating haemodialysis water.
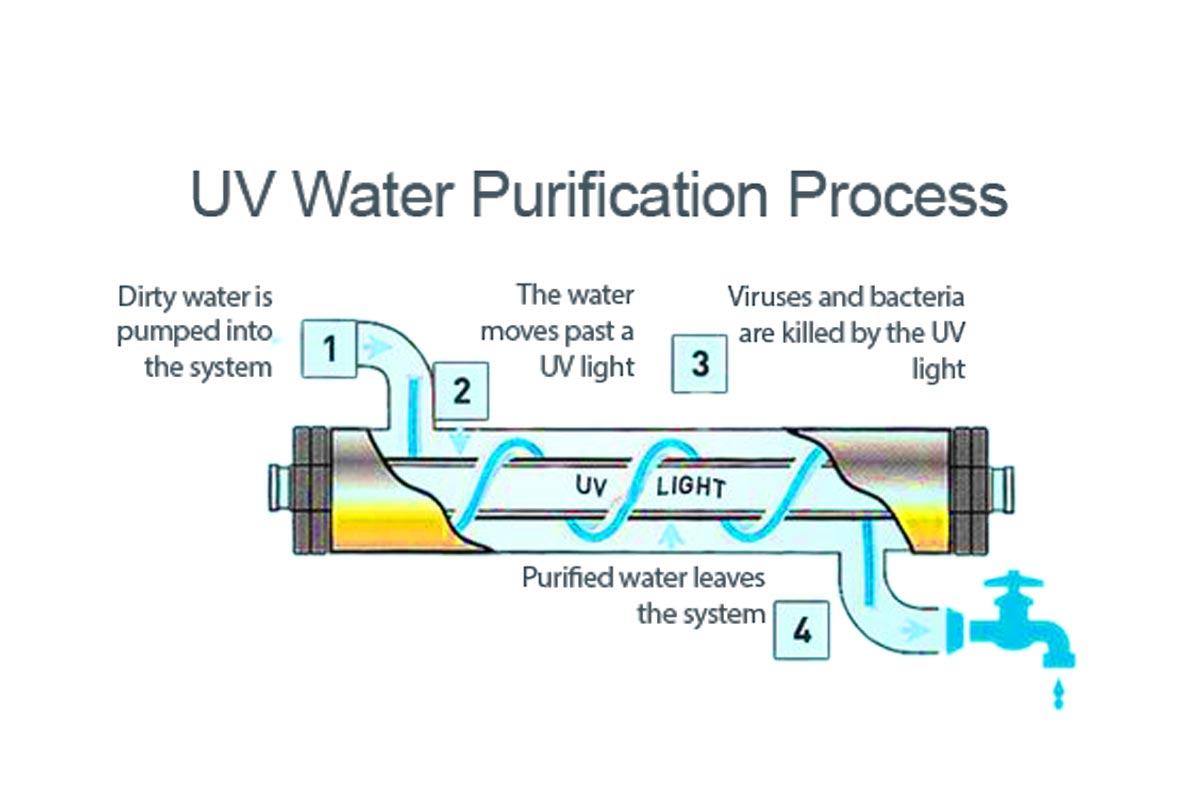
VIII. Ultraviolet Disinfection
Ultraviolet disinfection is one of the commonly employed methods today. Its sterilisation mechanism involves destroying the nucleic acids—the genetic material—of bacteria, thereby preventing reproduction. The most significant reaction occurs when pyrimidine bases within the nucleic acid molecules form dimers. Typically, artificial ultraviolet energy at a wavelength of 253.7nm is generated using low-pressure mercury discharge lamps (germicidal lamps). The principle of ultraviolet germicidal lamps is identical to that of fluorescent lamps, except that the tube interior lacks fluorescent coating and is constructed from quartz glass with high ultraviolet transmittance. Ultraviolet systems are categorised by application into irradiation, immersion, and flow-through types.
IX. Biochemical Method
Biochemical water treatment harnesses naturally occurring bacteria and microorganisms to decompose organic matter in wastewater into harmless substances, thereby purifying the effluent. This method encompasses activated sludge processes, biofilm systems, biological oxidation towers, land treatment systems, and anaerobic biological treatment.
Biochemical water treatment flow:
Raw water → Bar screen → Equalisation tank → Contact oxidation tank → Sedimentation basin → Filtration → Disinfection → Effluent discharge.

 Analysis of the Structure and Working Principle of Ultrapure Water Equipment
Analysis of the Structure and Working Principle of Ultrapure Water Equipment
 Tips for Using Home Water Purifier Filter Cartridges
Tips for Using Home Water Purifier Filter Cartridges
 The Battle of Four Membranes: Microfiltration, Ultrafiltration, Nanofiltration, Reverse Osmosis—Which Reigns Supreme in Water Purification?
The Battle of Four Membranes: Microfiltration, Ultrafiltration, Nanofiltration, Reverse Osmosis—Which Reigns Supreme in Water Purification?
 Don't Pay for Stupidity! The Ultimate 2026 Water Purifier Guide: Selection, Pitfalls, and Maintenance All in One Article
Don't Pay for Stupidity! The Ultimate 2026 Water Purifier Guide: Selection, Pitfalls, and Maintenance All in One Article