Leachate from Landfills | Application Summary of 6 Membrane Technologies in Water Treatment
Membrane treatment generally refers to membrane separation technology, which is a method designed based on the principle of selective permeability of biological membranes to separate mixed samples containing different components. The membranes used in the separation process are high-polymer materials synthesised according to specific requirements, and the mixed samples to be separated can be either liquid or gas.
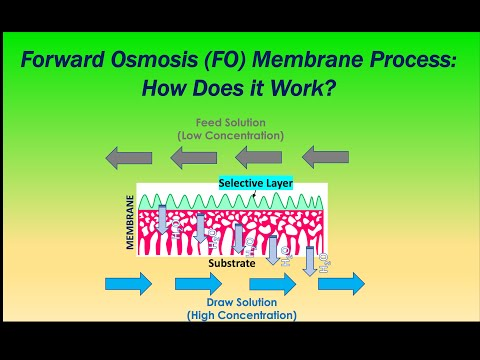
 Forward Osmosis (FO) Technology
Forward Osmosis (FO) Technology
The principle of forward osmosis (FO) involves using a semi-permeable membrane that allows only solvent molecules to pass through while blocking solute molecules, thereby separating the solvent from the solution. Under osmotic pressure, solvent molecules spontaneously migrate from the solvent side through the membrane into the solution side, which is known as the osmosis phenomenon, or ‘forward osmosis.’
Applications of Forward Osmosis Membranes in Water Treatment
1. Seawater Desalination
FO for seawater desalination is one of the most extensively studied fields. Early application studies were primarily documented in patents, but these studies were largely immature and lacked feasibility.
2. Industrial Wastewater Treatment
Early studies reported the use of FO membranes for treating low-concentration heavy metal wastewater, but due to severe fouling of the RO (reverse osmosis) membranes and rapid decline in flux, further development was not pursued.
3. Leachate Treatment
The Coffin Butte landfill in Corvallis, Oregon, USA, produces (2–4) × 10⁴ m³ of leachate annually. To meet land use water quality standards, the total dissolved solids (TDS) in the effluent must be reduced to below 100 mg/L.
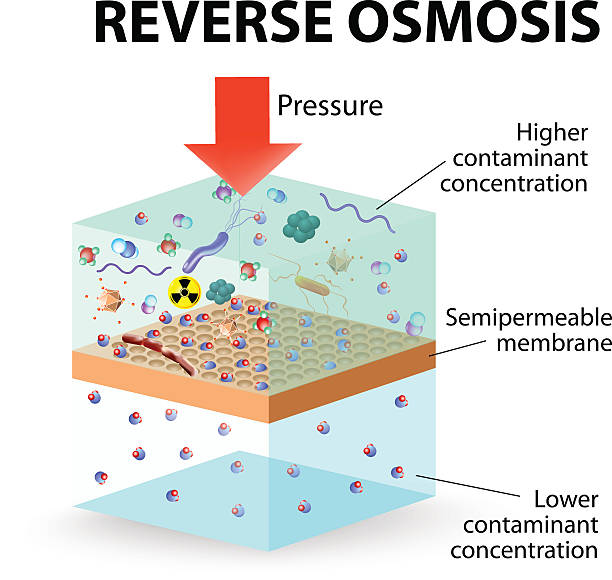
 Reverse Osmosis (RO) Technology
Reverse Osmosis (RO) Technology
Applications of Reverse Osmosis Membranes in Water Treatment
1. Conventional Applications in Water Treatment
Water is an indispensable material condition for human survival and production activities. Due to the increasing scarcity of freshwater resources, the global capacity of reverse osmosis water treatment systems has reached millions of tonnes per day.
2. Applications in Municipal Wastewater Treatment
Currently, the application of reverse osmosis membranes in advanced municipal wastewater treatment, particularly for secondary effluent reuse and recycled water reuse at wastewater treatment plants, has received significant attention.
3. Applications in Heavy Metal Wastewater Treatment
Conventional treatment methods for wastewater containing heavy metal ions merely transfer pollution, converting dissolved heavy metals into precipitates or more easily treatable forms. Their final disposal often involves landfilling, yet the risk of secondary pollution to groundwater and surface water environments from heavy metals persists long-term.
4. Applications in oily wastewater treatment
Oily wastewater is a widespread industrial wastewater. If directly discharged into water bodies, it forms an oil film on the water surface, obstructing oxygen dissolution and causing oxygen deficiency, biological death, and foul odours, severely polluting the ecological environment. Oil-containing produced water from oilfields with oil concentrations of 3.5 mg/L and total organic carbon (TOC) levels of 16–23 mg/L is treated to meet boiler water quality standards, and the treated water is reused as boiler feedwater for power plants.
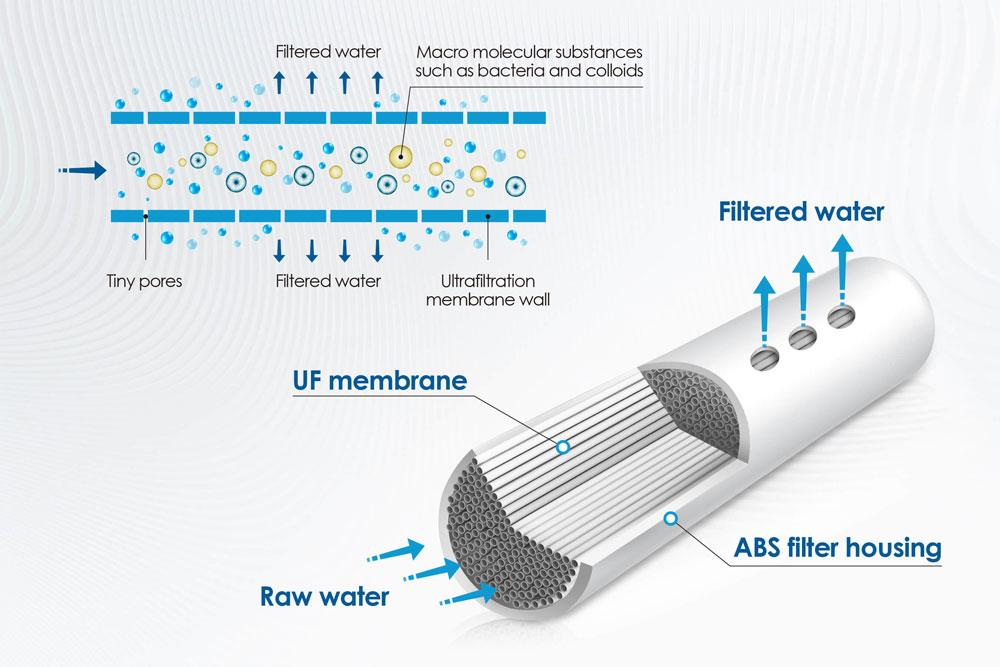
 Ultrafiltration (UF) and Microfiltration (MF) Technologies
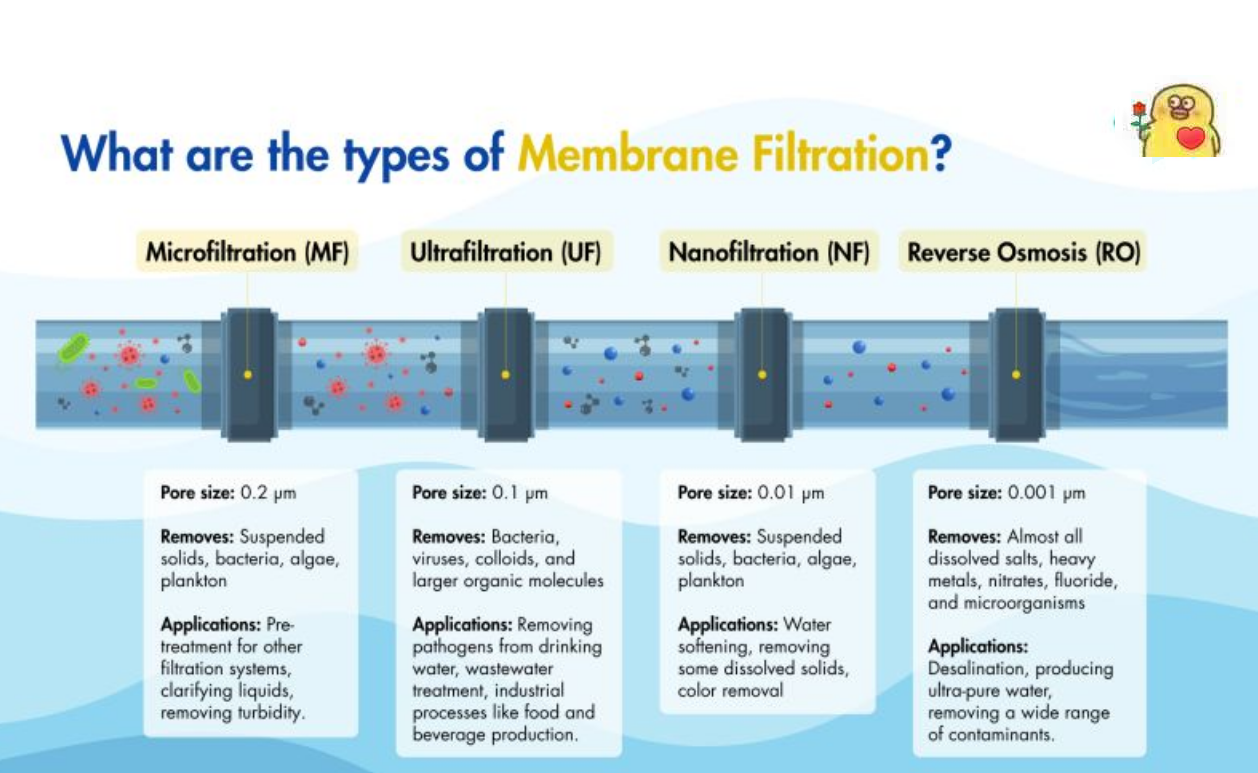
Basic Principles of Ultrafiltration (UF) and Microfiltration (MF)
Ultrafiltration (UF) and Microfiltration (MF) Technologies
Basic Principles of Ultrafiltration (UF) and Microfiltration (MF)
Ultrafiltration and microfiltration are both processes of liquid phase separation driven by static pressure differences. From a theoretical perspective, there is no fundamental difference between the two, as they are both sieve-pore separation processes. Under certain pressure conditions, when a mixed solution containing high-molecular-weight solutes and low-molecular-weight solutes flows over the membrane surface, the solvent and low-molecular-weight solutes (such as inorganic salts) smaller than the membrane pores pass through the membrane and are collected as permeate; high-molecular-weight solutes (such as organic colloids) larger than the membrane pores are retained by the membrane and recovered as concentrate. Membrane separation processes that can retain molecules with molecular weights above 500 and below 10⁶ are referred to as ultrafiltration; membrane separation processes that can only retain larger molecules (typically referred to as dispersed particles) are referred to as microfiltration.
 Applications of ultrafiltration and microfiltration membranes
Applications of ultrafiltration and microfiltration membranes
Ultrafiltration and microfiltration technologies can effectively remove particulate matter, including microorganisms such as cryptosporidium, giardia, bacteria, and viruses. They can also reduce disinfection by-products by lowering the concentration of disinfection by-product precursors and limiting the demand for oxidants during the disinfection process. However, their removal efficiency for organic matter in water is very low, typically below 20%. Ultrafiltration and microfiltration have a wide range of applications and can be used to treat water of varying quality.
Nanofiltration (NF) Membrane Technology
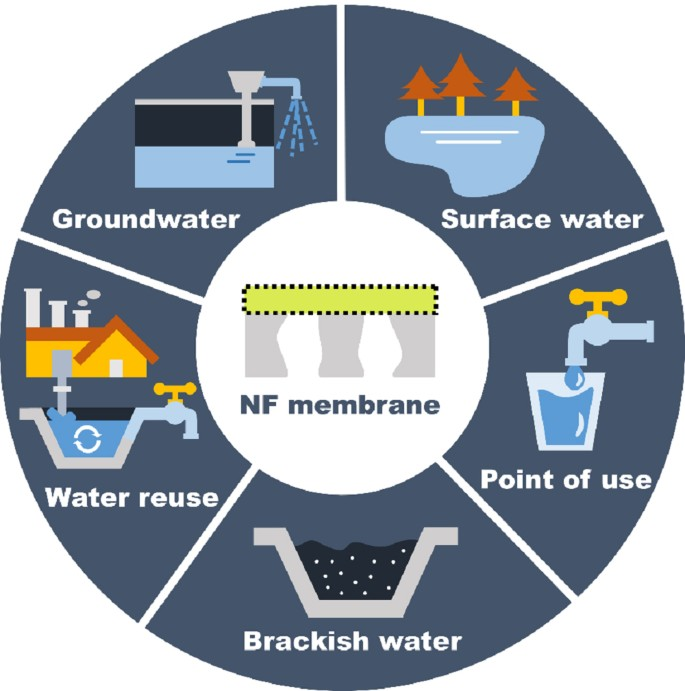
Nanofiltration (NF) is a novel molecular-level membrane separation technology and one of the current research hotspots in the global membrane separation field. NF membranes have pore sizes of over 1 nm, typically ranging from 1 to 2 nm; their retention performance for solutes lies between that of RO and UF membranes; RO membranes achieve very high removal rates for almost all solutes, but NF membranes only exhibit high removal rates for specific solutes. NF membranes can remove divalent and trivalent ions, organic compounds with Mn ≥ 200, as well as microorganisms, colloids, pyrogens, and viruses. A significant characteristic of NF membranes is that the membrane itself carries a charge. This is a key reason why NF membranes can achieve high desalination performance at very low pressures (as low as 0.5 MPa) and why membranes with a molecular weight cut-off of several hundred can still remove inorganic salts. It is also the primary reason for the relatively low operating costs of NF systems. NF is suitable for various saline water sources, with a water recovery rate generally ranging from 75% to 85%, and from 30% to 50% in seawater desalination. There is no discharge of acidic or alkaline wastewater.
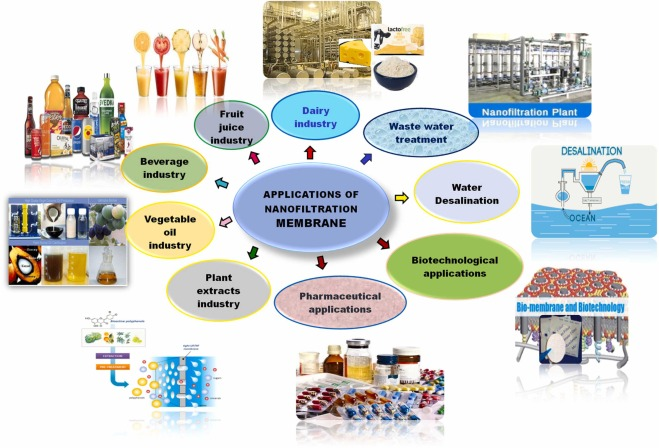 Applications of NF Membranes in Water Treatment
Applications of NF Membranes in Water Treatment
1. Application of Nanofiltration Membranes in Drinking Water
Nanofiltration operates at low pressure, making it the preferred process for drinking water production and advanced purification. Currently, most urban water sources are contaminated to varying degrees, and conventional treatment processes at water treatment plants have low removal efficiency for organic compounds in water. When chlorine is used for disinfection, it reacts with organic compounds in water to form halogenated by-products. A four-year follow-up study by Peltier et al. showed that after adopting a nanofiltration system, the DOC in water decreased to an average of 0.7 mgC/L, the residual chlorine content in the effluent decreased from 0.35 mg/L to 0.1 mg/L, and the formation of trihalomethanes (THMs) in the final effluent was reduced by 50% compared to when the nanofiltration system was not used. Additionally, the reduction in biodegradable dissolved organic carbon (BCOD) improved the biological stability of the treated water. Nanofiltration technology can remove the majority of Ca, Mg, and other ions, making desalination the most common application of nanofiltration technology. Membrane-based water treatment technology is comparable to conventional lime softening and ion exchange processes in terms of investment, operation, maintenance, and cost, but it offers advantages such as no sludge production, no regeneration requirements, complete removal of suspended solids and organic matter, simple operation, and minimal land use, with numerous application examples. Nanofiltration can be directly applied for the softening of groundwater, surface water, and wastewater, and can also serve as pre-treatment for reverse osmosis (RO), solar photovoltaic desalination systems, and other processes.
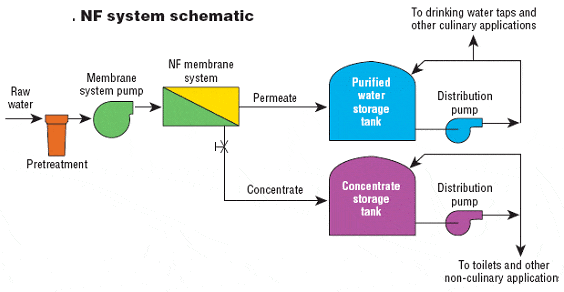
2. Application of nanofiltration membranes in seawater desalination
Seawater desalination refers to the process of reducing the salinity of seawater from 35,000 mg/L to below 500 mg/L to produce potable water.
3. Application of Nanofiltration Membranes in Wastewater Treatment
(1) Domestic Sewage
Domestic sewage is typically treated using a combination of biological degradation and chemical oxidation methods, but these methods require large amounts of oxidants and leave significant residues. Xue Gang et al. conducted small-scale experiments on hotel bathing wastewater using a combination of microflocculation fibre ball filtration, ultrafiltration, and nanofiltration processes. The ultrafiltration effluent quality meets the requirements for reuse in hotel toilet flushing and landscaping, while the nanofiltration effluent quality meets the national drinking water hygiene standards (GB5749.85), enabling reuse in higher-quality applications such as hotel laundry and bathing.
(2) Textile and dyeing wastewater:
Dyes in textile wastewater are difficult to remove using biological methods. Hassani studied the effects of concentration, pressure, total dissolved solids, and inorganic salt content on the retention performance of nanofiltration membranes for acidic, reactive, direct, and disperse dye solutions.
(3) Leather tanning wastewater
Leather tanning wastewater contains high concentrations of organic matter, sulphates, and chlorides, with the conductivity of the acid washing process waste liquid reaching 75 mS/cm. Bes-Pia used NF technology to recover leather tanning wastewater, with the high-concentration sulphate concentrate returned to the acid washing section and the chloride-containing permeate returned to the cracking reaction drum.
(4) Electroplating wastewater
Electroplating plants often generate large amounts of waste liquid. Despite complex treatment steps such as acidification, chemical neutralisation, sedimentation, and sludge separation, the resulting water has high salt content and cannot be reused.
(5) Paper mill wastewater
In the pulp and paper industry, processes such as pulping, bleaching, and paper production require large amounts of water. Achieving a (semi-)closed-loop water system is the optimal approach for pulp mills and paper mills to conserve water resources and reduce emissions. The effluent from the traditional activated sludge process still contains some coloured compounds, microorganisms, antibodies, and small amounts of biodegradable substances, as well as suspended solids, making it suitable only for the production of packaging paper and not for higher-grade paper. Additionally, this method cannot reduce the concentration of inorganic salts.
Koyuncu compared the practicality of two treatment processes: water → nanofiltration and paper mill wastewater → activated sludge → nanofiltration. The experiments showed that the effluent quality of the two methods was similar, but the second method had a better water flux, and the effluent could be used for high-grade paper. However, the nanofiltration effluent still contained a certain amount of monovalent salts, so a low-pressure reverse osmosis unit needed to be added to remove the salts to ensure the quality of the recycled water.
 Dialysis and electrodialysis technologies
Dialysis and electrodialysis technologies
Dialysis (Dialysis, abbreviated as D) is the process by which solutes move from the upstream side of a membrane to the downstream side under the influence of their own concentration gradient.
Dialysis was the first membrane separation technology to be discovered and studied, but due to inherent system limitations, the dialysis process is slow, inefficient, and lacks selectivity. Therefore, dialysis is primarily used to remove low-molecular-weight components from solutions containing multiple solutes, such as in haemodialysis, where the dialysis membrane replaces the kidneys to remove urea, creatinine, phosphates, and uric acid, thereby alleviating the condition of patients with renal failure and uremia.
Electrodialysis
Electrodialysis (ED) is a process that uses a direct current electric field to apply a potential difference as the driving force. By leveraging the selective permeability of ion exchange membranes to separate ions in a solution, electrolytes are removed from the solution, thereby achieving concentration, dilution, purification, and refinement of the solution.
Bipolar Membrane Technology
Introduction to Bipolar Membranes
A bipolar membrane (BPM) is a new type of membrane typically composed of a composite structure combining an anion exchange layer and a cation exchange layer. An additional third layer can be inserted between the anion and cation membranes to promote water dissociation, forming a three-layer structure comprising an anion exchange layer, a cation exchange layer, and an intermediate reaction layer. Under the influence of a direct current electric field, the bipolar membrane can dissociate water, producing H+ and OH- on the anion membrane and cation membrane sides, respectively.
Applications of Bipolar Membranes
1. Treatment of fluoride-containing waste liquids and recovery of valuable fluoride
In fluorocarbon industry and uranium industry (UF6) production, the mass fraction of fluoride and organic acids in the emitted waste gas and wastewater ranges from 50 to 500 × 10-6. Typically, KOH neutralisation is required to completely remove them. The resulting KF solution contains numerous heavy metals (such as uranium and arsenic) and trace radioactive substances. Additionally, Ca(OH)₂ must react with KF to regenerate KOH and produce insoluble waste. This method results in the loss of valuable fluorine and leaves users with the problem of how to dispose of Ca(OH)₂ waste containing radioactive substances. If bipolar membrane electro-osmotic dissociation technology is adopted, KF can be directly converted into HF and KOH, not only recovering high-value fluorine but also avoiding the use of lime and reducing the volume of waste residue.
2. Bipolar membranes for the purification and recovery of acidic and alkaline waste liquids
Industrial production generates a significant amount of acidic and alkaline waste liquids, such as ion exchange resin regeneration waste liquids, acid washing waste liquids, lead-acid battery waste liquids, and paper mill waste liquids. To reduce environmental pollution, these waste liquids must undergo necessary treatment before discharge, but the treatment processes are complex and costly. The bipolar membrane electrodialysis process offers an effective solution for treating such waste liquids. In 1986, China installed a combined electrodialysis and ion exchange system at the Zhejiang Province Post and Telecommunications Printing Plant to treat copper-containing wastewater. After treatment, the wastewater contained 100 mg/L of copper and had a pH of 6–7, meeting the permitted discharge standards.
3. Domestic wastewater treatment
Domestic wastewater is generally treated using a combination of biological degradation and chemical oxidation methods, but the amount of oxidising agent used is too high, resulting in excessive residues. If a nanofiltration stage is added between these processes, small molecules (relative molecular mass < 100) that can be degraded by microorganisms can pass through, while large molecules (relative molecular mass > 100) that cannot be degraded by microorganisms are retained. Large molecules are first treated with a chemical oxidiser and then undergo biological degradation, thereby fully utilising biological degradability, reducing the consumption of oxidants and activated carbon, and lowering the final residual content.
4. Drinking Water Purification
As water pollution worsens, people are increasingly concerned about drinking water quality. Experiments have proven that the bipolar membrane nanofiltration method can remove microtoxic by-products generated during disinfection, trace amounts of herbicides, pesticides, heavy metals, natural organic matter, hardness, sulphates, and nitrates. It also has the advantages of good and stable water quality, low chemical reagent consumption, small footprint, energy efficiency, and ease of management and maintenance.
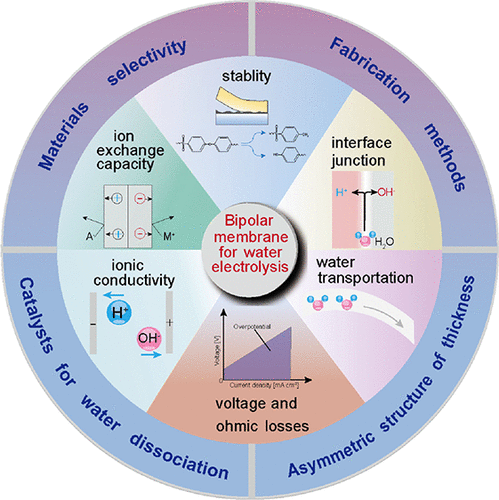
5. Treatment of Heavy Metal-Containing Wastewater
In electroplating and alloy production, large amounts of water are often used for rinsing, and these rinse waters contain high concentrations of heavy metals such as nickel, iron, copper, and zinc. To ensure these heavy metal-containing wastewater meets discharge standards, conventional measures involve treating the heavy metals into hydroxide precipitates for removal. If nanofiltration membrane technology is adopted, not only can over 90% of the wastewater be recovered and purified, but the concentration of heavy metal ions can also be increased by 10 times, making the concentrated heavy metals valuable for recycling.
6. Treatment of wastewater from the food industry
N-P type composite bipolar nanofiltration membranes have a significant separation effect on monovalent and divalent salts, significantly reducing the COD content in wastewater to meet environmental protection requirements.
7. Prospects for Bipolar Membranes
As a new type of membrane, bipolar membranes offer unique advantages, providing new approaches and solutions to long-standing technical challenges in environmental engineering. Continuing to develop high-performance bipolar membranes, improving membrane preparation processes, reducing production costs, conducting in-depth mechanistic studies on ion migration and water transport within membranes, researching high-performance bipolar membrane materials and their preparation, and expanding application areas hold profound significance.











 Analysis of the Structure and Working Principle of Ultrapure Water Equipment
Analysis of the Structure and Working Principle of Ultrapure Water Equipment
 Tips for Using Home Water Purifier Filter Cartridges
Tips for Using Home Water Purifier Filter Cartridges
 The Battle of Four Membranes: Microfiltration, Ultrafiltration, Nanofiltration, Reverse Osmosis—Which Reigns Supreme in Water Purification?
The Battle of Four Membranes: Microfiltration, Ultrafiltration, Nanofiltration, Reverse Osmosis—Which Reigns Supreme in Water Purification?
 Don't Pay for Stupidity! The Ultimate 2026 Water Purifier Guide: Selection, Pitfalls, and Maintenance All in One Article
Don't Pay for Stupidity! The Ultimate 2026 Water Purifier Guide: Selection, Pitfalls, and Maintenance All in One Article