The Application of Activated Carbon in Water Treatment: You Might Not Know Unless You Ask!
1. Introduction
According to statistics, China produces approximately 8×10⁸ m³ of industrial wastewater annually, which not only contains highly toxic components such as cyanide but also metal ions like chromium, zinc, and nickel. There are various methods for treating wastewater, including chemical precipitation, electrolysis, and membrane filtration. This article focuses on the activated carbon adsorption method. Activated carbon has an enormous surface area and possesses strong physical and chemical adsorption capabilities. Therefore, the activated carbon adsorption method is widely applied in wastewater treatment, offering advantages such as high efficiency and excellent results.

2. Activated Carbon
Activated carbon is a type of carbon that has undergone special processing, featuring countless tiny pores and an enormous surface area. Each gram of activated carbon has a surface area of 500–1,500 square metres. Activated carbon possesses strong physical and chemical adsorption capabilities and also has detoxification properties. Detoxification involves utilising its vast surface area to adsorb toxins into the microporous structure of activated carbon, thereby preventing their absorption. Additionally, activated carbon can bind with various chemical substances, thereby preventing their absorption.
2.1 Classification of Activated Carbon
There are many types of activated carbon used in production. It is generally produced in powder or granular form.
Powdered activated carbon has strong adsorption capacity, is easy to prepare, and is relatively inexpensive, but it is difficult to regenerate and generally cannot be reused. Granular activated carbon is more expensive but can be regenerated and reused, and it offers better working conditions and easier operation and management during use. Therefore, granular activated carbon is more commonly used in water treatment.

2.2 Activated Carbon Adsorption
Activated carbon adsorption refers to the use of the solid surface of activated carbon to adsorb one or more substances in water, thereby achieving the purpose of water purification.
2.3 Factors Affecting Activated Carbon Adsorption
Adsorption capacity and adsorption rate are the primary indicators for evaluating the adsorption process.
Adsorption capacity is measured by the amount of adsorption. Adsorption rate refers to the amount of substance adsorbed by a unit weight of adsorbent per unit time. In water treatment, the adsorption rate determines the contact time required between wastewater and the adsorbent. The adsorption capacity of activated carbon is related to the size and structure of its pores. Generally, the smaller the particles, the faster the pore diffusion rate, and the stronger the adsorption capacity of the activated carbon.
The pH value and temperature of wastewater also affect the adsorption capacity of activated carbon. Activated carbon generally has a higher adsorption capacity under acidic conditions than under alkaline conditions. Adsorption reactions are typically exothermic, so lower temperatures are advantageous for adsorption reactions. Of course, the adsorption capacity of activated carbon is also related to the concentration of wastewater. At a constant temperature, the adsorption capacity of activated carbon increases with the equilibrium concentration of the adsorbed substance.
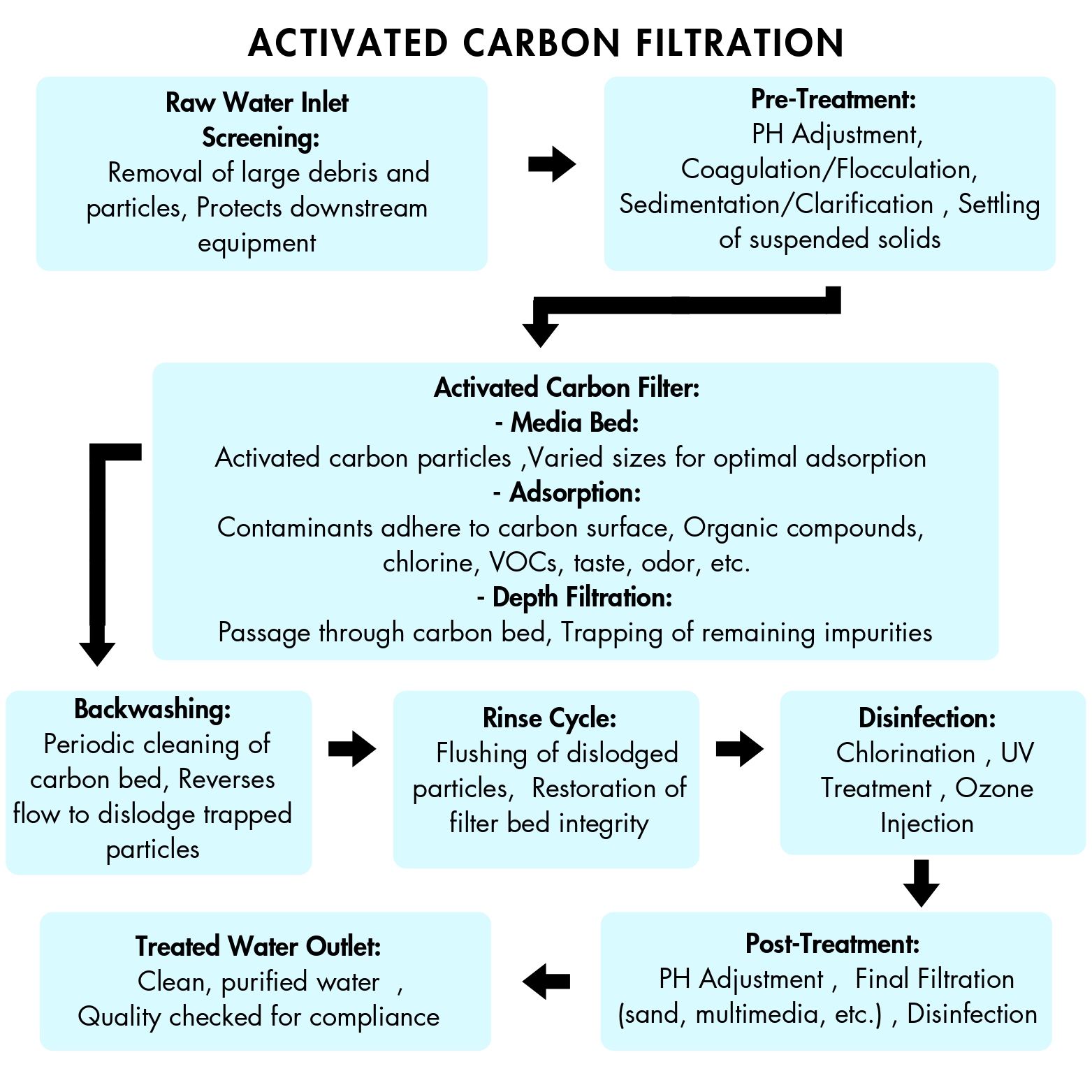
3. Application of activated carbon in wastewater treatment
Due to the high pre-treatment requirements for water and the high cost of activated carbon, it is primarily used in wastewater treatment to remove trace pollutants, achieving the goal of deep purification.
3.1 Activated Carbon Treatment of Chromium-Containing Wastewater
Chromium is a widely used metal raw material in electroplating. In wastewater, hexavalent chromium exists in different forms depending on the pH value.
Activated carbon has a highly developed microporous structure and a large specific surface area, endowing it with strong physical adsorption capacity, enabling it to effectively adsorb Cr(VI) from wastewater. The surface of activated carbon contains numerous oxygen-containing functional groups such as hydroxyl (-OH) and carboxyl (-COOH) groups, which possess electrostatic adsorption capabilities and exert chemical adsorption effects on Cr(VI). It can be fully utilised for treating Cr(VI) in electroplating wastewater, with the adsorbed wastewater meeting national emission standards.
Experiments indicate that when the Cr(VI) mass concentration in the solution is 50 mg/L, pH = 3, and adsorption time is 1.5 hours, the adsorption performance of activated carbon and the removal rate of Cr(VI) both achieve optimal results.
Therefore, the process of treating chromium-containing wastewater using activated carbon is the result of a combination of physical adsorption, chemical adsorption, and chemical reduction of Cr(VI) in the solution by activated carbon. Activated carbon treatment of chromium-containing wastewater exhibits stable adsorption performance, high treatment efficiency, low operating costs, and significant social and economic benefits. 3.2 Activated carbon treatment of cyanide-containing wastewater In industrial production, industries such as wet extraction of gold and silver, chemical fibre production, coking, synthetic ammonia production, electroplating, and coal gas production all use cyanides or produce cyanide by-products, resulting in the inevitable discharge of a certain amount of cyanide-containing wastewater during production.

3.2 Activated Carbon Treatment of Cyanide-Containing Wastewater
In industrial production, industries such as wet extraction of gold and silver, chemical fibre production, coking, synthetic ammonia production, electroplating, and gas production all use cyanide or produce cyanide as a by-product, resulting in the inevitable discharge of a certain amount of cyanide-containing wastewater during production.
Activated carbon has been used for wastewater purification for a considerable period of time, and there are increasing reports in the literature on its application in treating cyanide-containing wastewater. However, due to the low adsorption capacity of CN⁻ and HCN on activated carbon, typically ranging from 3 mg CN/g AC to 8 mg CN/g AC (depending on the type), it is not cost-effective for treatment purposes.
3.3 Activated Carbon Treatment of Mercury-Containing Wastewater
Activated carbon has the ability to adsorb mercury and mercury compounds, but its adsorption capacity is limited, making it suitable only for treating wastewater with low mercury content. If the mercury concentration is high, chemical precipitation methods can be used first to reduce the mercury content to approximately 1 mg/L, or up to 2–3 mg/L at maximum, followed by further treatment with activated carbon.
3.4 Activated Carbon Treatment of Phenol-Containing Wastewater
Phenol-containing wastewater is widely sourced from petrochemical plants, resin plants, coking plants, and refining chemical plants. Experimental evidence shows that activated carbon has good adsorption performance for phenol; however, increased temperature is detrimental to adsorption, reducing adsorption capacity; yet, it shortens the time required to reach adsorption equilibrium. There are optimal values for activated carbon dosage and adsorption time. Under acidic and neutral conditions, the removal efficiency remains relatively stable; however, under strongly alkaline conditions, the phenol removal efficiency drops sharply, with stronger alkalinity leading to poorer adsorption performance.
3.5 Activated Carbon Treatment of Methanol-Containing Wastewater
Activated carbon can adsorb methanol, but its adsorption capacity is weak, making it suitable only for treating wastewater with low methanol content. Engineering operation results show that the COD of the mixed solution can be reduced from 40 mg/L to below 12 mg/L, with a methanol removal rate of 93.16% to 100%, and the effluent water quality meets the requirements for reuse in the boiler desalination water system feedwater.

3.6 Advanced Treatment in Refineries
Refinery wastewater containing oil is first subjected to oil separation, flotation, and biological treatment, followed by sand filtration and activated carbon filtration for advanced treatment. The phenol content in the wastewater is reduced from 0.1 mg/L (after biological treatment) to 0.005 mg/L, cyanide from 0.19 mg/L to 0.048 mg/L, COD from 85 mg/L to 18 mg/L.
4. Prospects
With the advancement of science and technology and the special requirements of wastewater treatment, research on activated carbon has evolved from studying its pore structure and specific surface area to investigating the influence of surface functional groups on its adsorption performance.
For example, activated carbon fibres (ACF) have recently garnered significant attention from researchers in wastewater treatment. Their diameter typically ranges from 5 to 20 μm, and their preparation principle is similar to that of traditional activated carbon, involving the activation of fibrous carbon at temperatures above 800°C using steam or carbon dioxide.

The pore structure of fibrous activated carbon is primarily microporous, with few mesopores and almost no macropores, achieving a specific surface area of up to 2,500 m²/g. It exhibits characteristics such as rapid adsorption and desorption rates, high adsorption capacity, and excellent conductivity.
Experiments show that the adsorption capacity of ACF for phenol is 248 mg/g. After adsorption saturation, the adsorption capacity remains almost unchanged after multiple regenerations, demonstrating superior adsorption performance compared to activated carbon. At room temperature, under acidic or neutral conditions, adding 0.5 g of activated carbon fibre to 100 mL of phenol-containing simulated wastewater with a concentration of 282 mg/L, followed by constant-temperature shaking for 30 minutes, achieves a phenol removal rate of 91%.
Recently, it has been discovered that activated carbon not only possesses adsorption properties but also exhibits catalytic properties. As a result, catalytic oxidation methods have gained increasing attention, and research in this area continues to deepen. To enhance treatment efficiency, studies on the catalytic oxidation mechanism have been conducted to modify the surface structure of activated carbon, enhance its capabilities, and identify ideal adsorbents.

5. Conclusion
Currently, the use of activated carbon adsorption methods for wastewater treatment in China is in its initial development stage. Some related theories and technologies are still not fully mature. Additionally, in China, the supply of activated carbon is currently tight, regeneration equipment is scarce, and regeneration costs are high, limiting the widespread use of activated carbon. Different applications require activated carbon with different functionalities. Existing activated carbon products cannot meet new requirements, so the continuous development of new activated carbon products is of great importance. Therefore, it requires the active participation of professionals and strong government support, adopting interdisciplinary and integrated research methods to advance activated carbon wastewater treatment technology toward a more scientific and promising direction.

 Analysis of the Structure and Working Principle of Ultrapure Water Equipment
Analysis of the Structure and Working Principle of Ultrapure Water Equipment
 Tips for Using Home Water Purifier Filter Cartridges
Tips for Using Home Water Purifier Filter Cartridges
 The Battle of Four Membranes: Microfiltration, Ultrafiltration, Nanofiltration, Reverse Osmosis—Which Reigns Supreme in Water Purification?
The Battle of Four Membranes: Microfiltration, Ultrafiltration, Nanofiltration, Reverse Osmosis—Which Reigns Supreme in Water Purification?
 Don't Pay for Stupidity! The Ultimate 2026 Water Purifier Guide: Selection, Pitfalls, and Maintenance All in One Article
Don't Pay for Stupidity! The Ultimate 2026 Water Purifier Guide: Selection, Pitfalls, and Maintenance All in One Article